 Found 612 hits of ic50 data for polymerid = 2338,50001334
Found 612 hits of ic50 data for polymerid = 2338,50001334 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50133435

(4-(2-Chloro-phenyl)-1-ethyl-6-methyl-1,4-dihydro-p...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)OC(C)C |c:4,10| Show InChI InChI=1S/C20H22ClNO6/c1-5-22-11(4)14(20(27)28-10(2)3)15(12-8-6-7-9-13(12)21)16(18(23)24)17(22)19(25)26/h6-10,15H,5H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit muscular GPb by NMR binding assay |
J Med Chem 55: 1287-95 (2012)
Article DOI: 10.1021/jm201439b
BindingDB Entry DOI: 10.7270/Q21J9BS9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135554

(4-{3-[(4-Nitro-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C24H15N3O8/c28-22(20-11-15(27(33)34)7-8-25-20)26-19-9-13-3-1-2-4-14(13)10-21(19)35-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133440
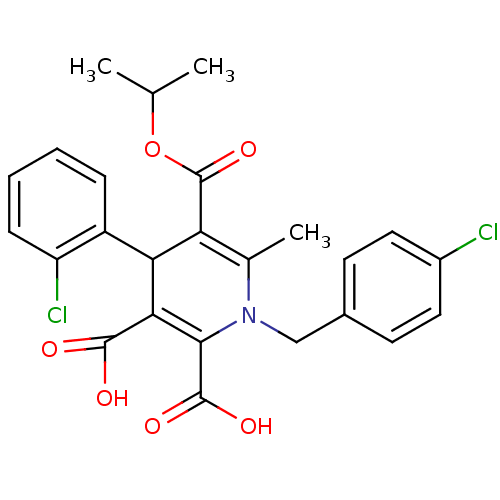
(1-(4-Chloro-benzyl)-4-(2-chloro-phenyl)-6-methyl-1...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2ccc(Cl)cc2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,22| Show InChI InChI=1S/C25H23Cl2NO6/c1-13(2)34-25(33)19-14(3)28(12-15-8-10-16(26)11-9-15)22(24(31)32)21(23(29)30)20(19)17-6-4-5-7-18(17)27/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50497973

(CHEMBL3323454)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1cc2ccccc2cc1CC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C30H36N2O5/c1-17-12-18(2)26(19(3)13-17)31-25(33)16-23-14-21-10-8-9-11-22(21)15-24(23)28(34)32-27(29(35)36)20(4)37-30(5,6)7/h8-15,20,27H,16H2,1-7H3,(H,31,33)(H,32,34)(H,35,36)/t20-,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase a assessed as inhibition of release of phosphate from glucose-1-phosphate after 30 mins by spectro... |
Eur J Med Chem 84: 584-94 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.063
BindingDB Entry DOI: 10.7270/Q2B85C38 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133443

(4-(2-Chloro-phenyl)-1-ethyl-5-isobutylcarbamoyl-6-...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)NCC(C)C |c:4,10| Show InChI InChI=1S/C21H25ClN2O5/c1-5-24-12(4)15(19(25)23-10-11(2)3)16(13-8-6-7-9-14(13)22)17(20(26)27)18(24)21(28)29/h6-9,11,16H,5,10H2,1-4H3,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135558
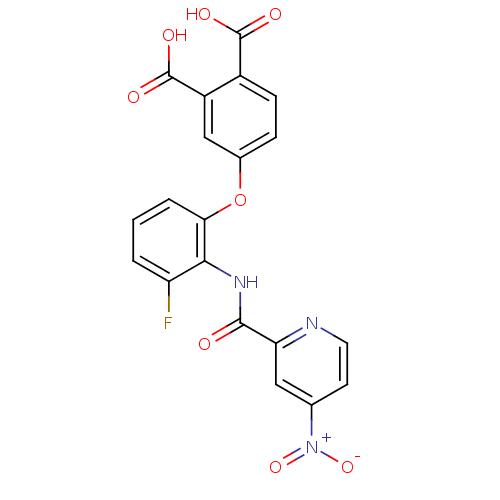
(4-{3-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-14-2-1-3-16(32-11-4-5-12(19(26)27)13(9-11)20(28)29)17(14)23-18(25)15-8-10(24(30)31)6-7-22-15/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133442

(4-(2-Chloro-phenyl)-6-methyl-1-(3-nitro-benzyl)-1,...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2cccc(c2)[N+]([O-])=O)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,24| Show InChI InChI=1S/C25H23ClN2O8/c1-13(2)36-25(33)19-14(3)27(12-15-7-6-8-16(11-15)28(34)35)22(24(31)32)21(23(29)30)20(19)17-9-4-5-10-18(17)26/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133429
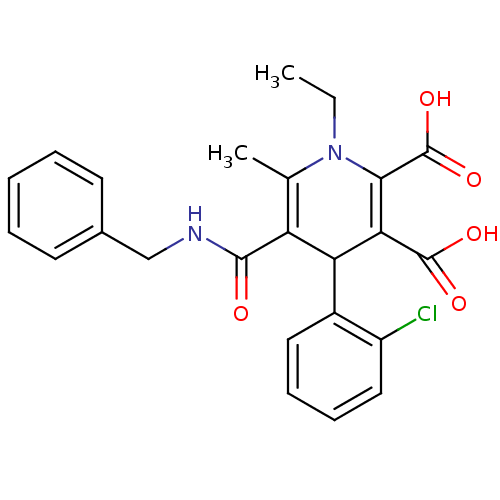
(5-Benzylcarbamoyl-4-(2-chloro-phenyl)-1-ethyl-6-me...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)NCc1ccccc1 |c:4,10| Show InChI InChI=1S/C24H23ClN2O5/c1-3-27-14(2)18(22(28)26-13-15-9-5-4-6-10-15)19(16-11-7-8-12-17(16)25)20(23(29)30)21(27)24(31)32/h4-12,19H,3,13H2,1-2H3,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133438

(4-(2-Chloro-phenyl)-1-(3,4-dimethoxy-benzyl)-6-met...)Show SMILES COc1ccc(CN2C(C)=C(C(C(C(O)=O)=C2C(O)=O)c2ccccc2Cl)C(=O)OC(C)C)cc1OC |c:9,15| Show InChI InChI=1S/C27H28ClNO8/c1-14(2)37-27(34)21-15(3)29(13-16-10-11-19(35-4)20(12-16)36-5)24(26(32)33)23(25(30)31)22(21)17-8-6-7-9-18(17)28/h6-12,14,22H,13H2,1-5H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135565
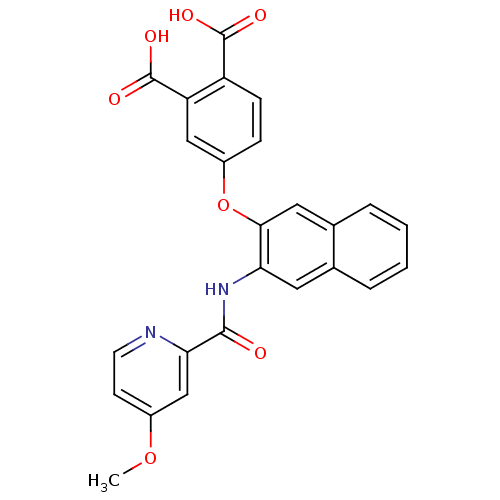
(4-{3-[(4-Methoxy-pyridine-2-carbonyl)-amino]-napht...)Show SMILES COc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O7/c1-33-16-8-9-26-21(13-16)23(28)27-20-10-14-4-2-3-5-15(14)11-22(20)34-17-6-7-18(24(29)30)19(12-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135553
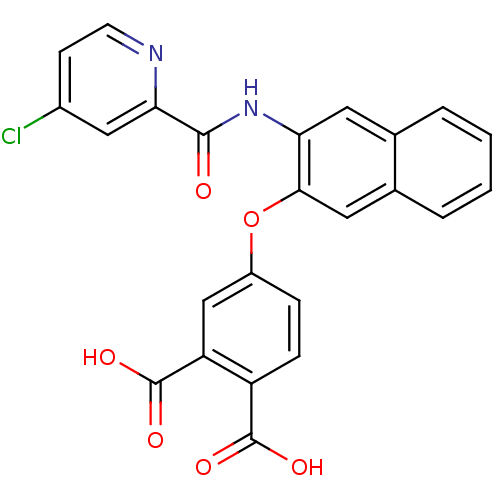
(4-{3-[(4-Chloro-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C24H15ClN2O6/c25-15-7-8-26-20(11-15)22(28)27-19-9-13-3-1-2-4-14(13)10-21(19)33-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133444
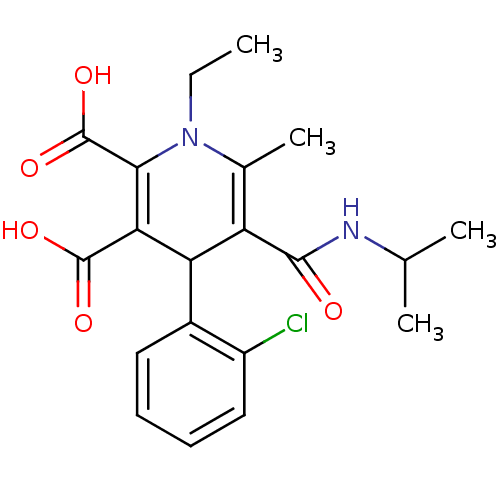
(4-(2-Chloro-phenyl)-1-ethyl-5-isopropylcarbamoyl-6...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)NC(C)C |c:4,10| Show InChI InChI=1S/C20H23ClN2O5/c1-5-23-11(4)14(18(24)22-10(2)3)15(12-8-6-7-9-13(12)21)16(19(25)26)17(23)20(27)28/h6-10,15H,5H2,1-4H3,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136445
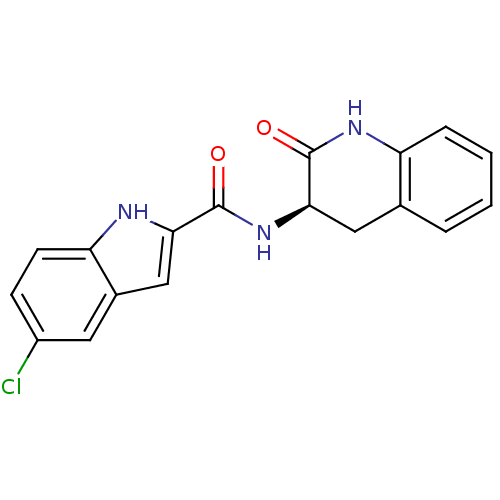
(5-Chloro-1H-indole-2-carboxylic acid ((R)-2-oxo-1,...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)N[C@@H]1Cc2ccccc2NC1=O Show InChI InChI=1S/C18H14ClN3O2/c19-12-5-6-14-11(7-12)9-15(20-14)17(23)22-16-8-10-3-1-2-4-13(10)21-18(16)24/h1-7,9,16,20H,8H2,(H,21,24)(H,22,23)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133433

(4-(2-Chloro-phenyl)-1-ethyl-5-ethylcarbamoyl-6-met...)Show SMILES CCNC(=O)C1=C(C)N(CC)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:5,14| Show InChI InChI=1S/C19H21ClN2O5/c1-4-21-17(23)13-10(3)22(5-2)16(19(26)27)15(18(24)25)14(13)11-8-6-7-9-12(11)20/h6-9,14H,4-5H2,1-3H3,(H,21,23)(H,24,25)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50382964
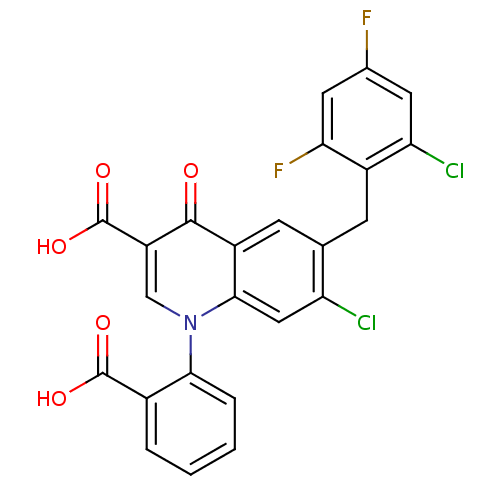
(CHEMBL2030481)Show SMILES OC(=O)c1ccccc1-n1cc(C(O)=O)c(=O)c2cc(Cc3c(F)cc(F)cc3Cl)c(Cl)cc12 Show InChI InChI=1S/C24H13Cl2F2NO5/c25-17-9-21-15(6-11(17)5-14-18(26)7-12(27)8-19(14)28)22(30)16(24(33)34)10-29(21)20-4-2-1-3-13(20)23(31)32/h1-4,6-10H,5H2,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit muscular GPb by NMR binding assay |
J Med Chem 55: 1287-95 (2012)
Article DOI: 10.1021/jm201439b
BindingDB Entry DOI: 10.7270/Q21J9BS9 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135562
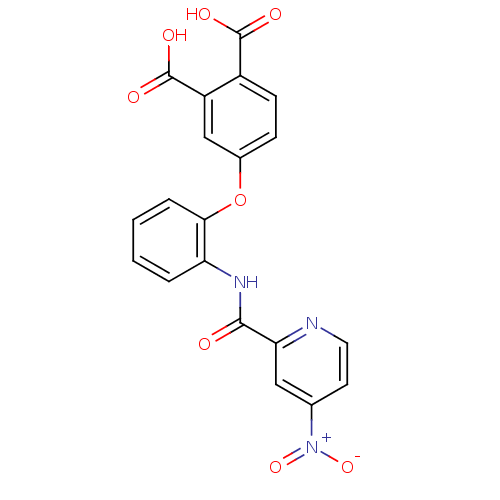
(4-{2-[(4-Nitro-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H13N3O8/c24-18(16-9-11(23(29)30)7-8-21-16)22-15-3-1-2-4-17(15)31-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133430

(1-Benzyl-4-(2-chloro-phenyl)-6-methyl-1,4-dihydro-...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2ccccc2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,21| Show InChI InChI=1S/C25H24ClNO6/c1-14(2)33-25(32)19-15(3)27(13-16-9-5-4-6-10-16)22(24(30)31)21(23(28)29)20(19)17-11-7-8-12-18(17)26/h4-12,14,20H,13H2,1-3H3,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135557
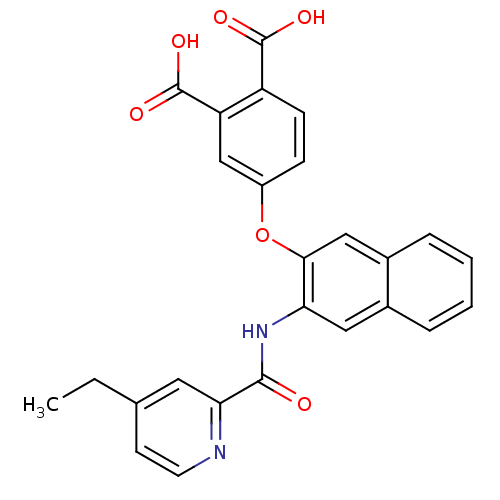
(4-{3-[(4-Ethyl-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES CCc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C26H20N2O6/c1-2-15-9-10-27-22(11-15)24(29)28-21-12-16-5-3-4-6-17(16)13-23(21)34-18-7-8-19(25(30)31)20(14-18)26(32)33/h3-14H,2H2,1H3,(H,28,29)(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133431
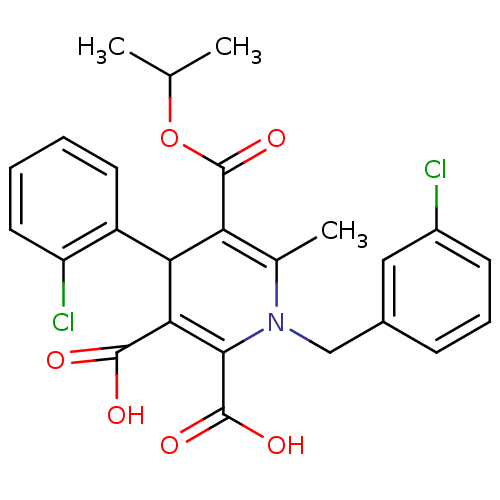
(1-(3-Chloro-benzyl)-4-(2-chloro-phenyl)-6-methyl-1...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2cccc(Cl)c2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,22| Show InChI InChI=1S/C25H23Cl2NO6/c1-13(2)34-25(33)19-14(3)28(12-15-7-6-8-16(26)11-15)22(24(31)32)21(23(29)30)20(19)17-9-4-5-10-18(17)27/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136451

(5-Chloro-1H-indole-2-carboxylic acid (1-methyl-2-o...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H16ClN3O2/c1-23-17-5-3-2-4-11(17)9-16(19(23)25)22-18(24)15-10-12-8-13(20)6-7-14(12)21-15/h2-8,10,16,21H,9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133441
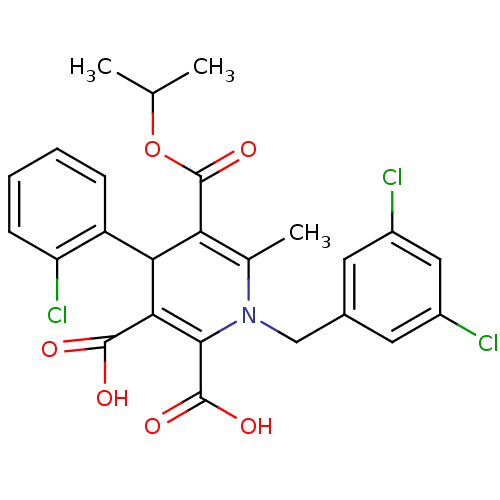
(4-(2-Chloro-phenyl)-1-(3,5-dichloro-benzyl)-6-meth...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2cc(Cl)cc(Cl)c2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,23| Show InChI InChI=1S/C25H22Cl3NO6/c1-12(2)35-25(34)19-13(3)29(11-14-8-15(26)10-16(27)9-14)22(24(32)33)21(23(30)31)20(19)17-6-4-5-7-18(17)28/h4-10,12,20H,11H2,1-3H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136437

(5-Chloro-1H-indole-2-carboxylic acid (2-oxo-1,2,3,...)Show InChI InChI=1S/C18H14ClN3O2/c19-12-5-6-14-11(7-12)9-15(20-14)17(23)22-16-8-10-3-1-2-4-13(10)21-18(16)24/h1-7,9,16,20H,8H2,(H,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136447
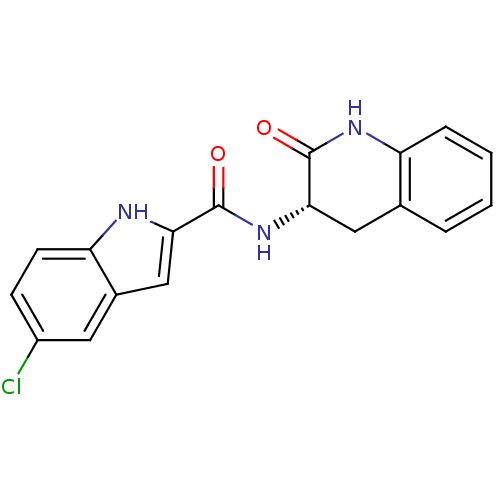
(5-Chloro-1H-indole-2-carboxylic acid ((S)-2-oxo-1,...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)N[C@H]1Cc2ccccc2NC1=O Show InChI InChI=1S/C18H14ClN3O2/c19-12-5-6-14-11(7-12)9-15(20-14)17(23)22-16-8-10-3-1-2-4-13(10)21-18(16)24/h1-7,9,16,20H,8H2,(H,21,24)(H,22,23)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133432

(4-(2-Chloro-phenyl)-1-(3,4-dichloro-benzyl)-6-meth...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2ccc(Cl)c(Cl)c2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,23| Show InChI InChI=1S/C25H22Cl3NO6/c1-12(2)35-25(34)19-13(3)29(11-14-8-9-17(27)18(28)10-14)22(24(32)33)21(23(30)31)20(19)15-6-4-5-7-16(15)26/h4-10,12,20H,11H2,1-3H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50497969
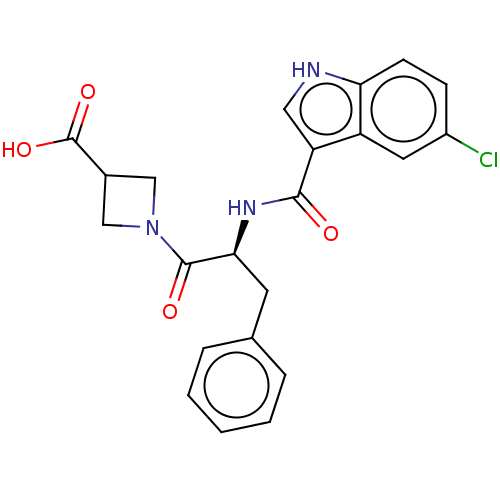
(CHEMBL3323453)Show SMILES OC(=O)C1CN(C1)C(=O)[C@H](Cc1ccccc1)NC(=O)c1c[nH]c2ccc(Cl)cc12 |r| Show InChI InChI=1S/C22H20ClN3O4/c23-15-6-7-18-16(9-15)17(10-24-18)20(27)25-19(8-13-4-2-1-3-5-13)21(28)26-11-14(12-26)22(29)30/h1-7,9-10,14,19,24H,8,11-12H2,(H,25,27)(H,29,30)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle glycogen phosphorylase a assessed as inhibition of release of phosphate from glucose-1-phosphate after 30 mins by spectro... |
Eur J Med Chem 84: 584-94 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.063
BindingDB Entry DOI: 10.7270/Q2B85C38 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133447

(4-(2-Chloro-phenyl)-6-methyl-1-(2,2,2-trifluoro-et...)Show SMILES CC(C)OC(=O)C1=C(C)N(CC(F)(F)F)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,18| Show InChI InChI=1S/C20H19ClF3NO6/c1-9(2)31-19(30)13-10(3)25(8-20(22,23)24)16(18(28)29)15(17(26)27)14(13)11-6-4-5-7-12(11)21/h4-7,9,14H,8H2,1-3H3,(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136424
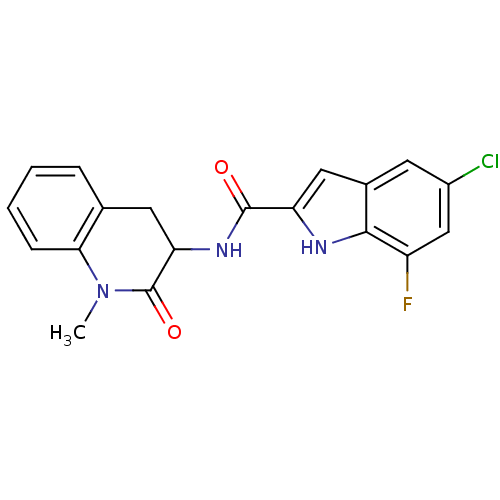
(5-Chloro-7-fluoro-1H-indole-2-carboxylic acid (1-m...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cc(Cl)cc(F)c2[nH]1 Show InChI InChI=1S/C19H15ClFN3O2/c1-24-16-5-3-2-4-10(16)7-15(19(24)26)23-18(25)14-8-11-6-12(20)9-13(21)17(11)22-14/h2-6,8-9,15,22H,7H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136435

(5-Chloro-1H-indole-2-carboxylic acid (2-oxo-2,3,4,...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NC1CCc2ccccc2NC1=O Show InChI InChI=1S/C19H16ClN3O2/c20-13-6-8-15-12(9-13)10-17(21-15)19(25)23-16-7-5-11-3-1-2-4-14(11)22-18(16)24/h1-4,6,8-10,16,21H,5,7H2,(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136418

(1H-Indole-2-carboxylic acid (1-methyl-2-oxo-1,2,3,...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2ccccc2[nH]1 Show InChI InChI=1S/C19H17N3O2/c1-22-17-9-5-3-7-13(17)11-16(19(22)24)21-18(23)15-10-12-6-2-4-8-14(12)20-15/h2-10,16,20H,11H2,1H3,(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135560
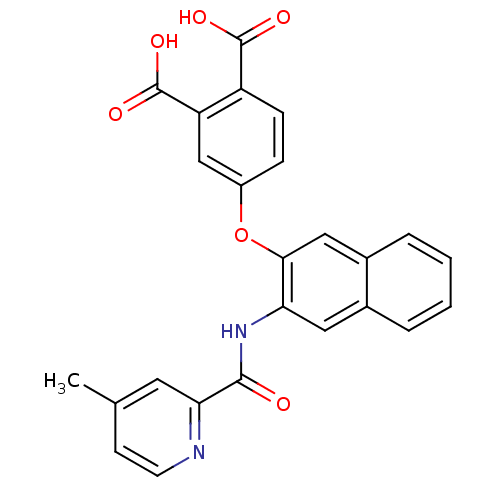
(4-{3-[(4-Methyl-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES Cc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O6/c1-14-8-9-26-21(10-14)23(28)27-20-11-15-4-2-3-5-16(15)12-22(20)33-17-6-7-18(24(29)30)19(13-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136428
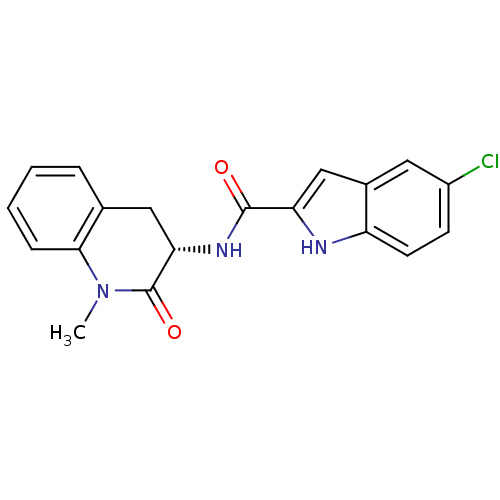
(5-Chloro-1H-indole-2-carboxylic acid ((S)-1-methyl...)Show SMILES CN1C(=O)[C@H](Cc2ccccc12)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H16ClN3O2/c1-23-17-5-3-2-4-11(17)9-16(19(23)25)22-18(24)15-10-12-8-13(20)6-7-14(12)21-15/h2-8,10,16,21H,9H2,1H3,(H,22,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136448

(5-Chloro-1H-indole-2-carboxylic acid ((R)-1-methyl...)Show SMILES CN1C(=O)[C@@H](Cc2ccccc12)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H16ClN3O2/c1-23-17-5-3-2-4-11(17)9-16(19(23)25)22-18(24)15-10-12-8-13(20)6-7-14(12)21-15/h2-8,10,16,21H,9H2,1H3,(H,22,24)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135550
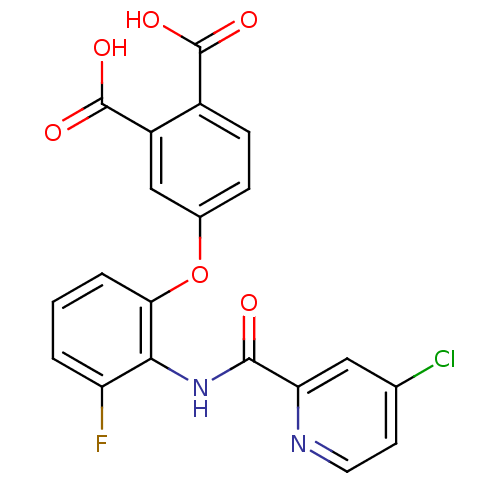
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136416
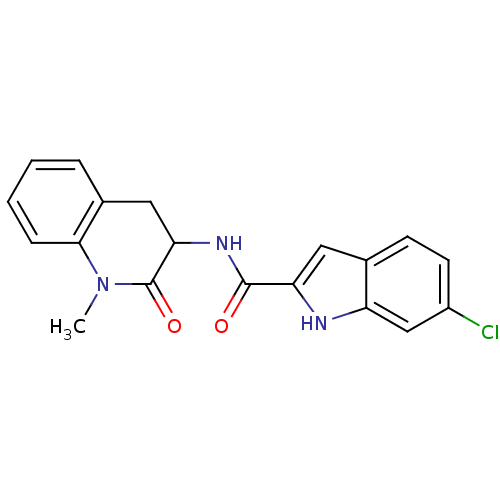
(6-Chloro-1H-indole-2-carboxylic acid (1-methyl-2-o...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2ccc(Cl)cc2[nH]1 Show InChI InChI=1S/C19H16ClN3O2/c1-23-17-5-3-2-4-12(17)9-16(19(23)25)22-18(24)15-8-11-6-7-13(20)10-14(11)21-15/h2-8,10,16,21H,9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136440

(5-Bromo-1H-indole-2-carboxylic acid (1-methyl-2-ox...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cc(Br)ccc2[nH]1 Show InChI InChI=1S/C19H16BrN3O2/c1-23-17-5-3-2-4-11(17)9-16(19(23)25)22-18(24)15-10-12-8-13(20)6-7-14(12)21-15/h2-8,10,16,21H,9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135567
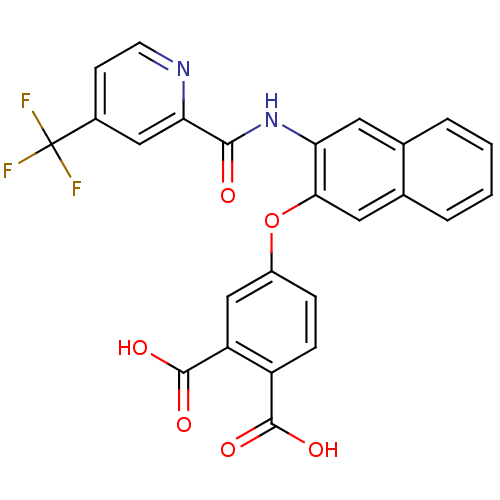
(4-{3-[(4-Trifluoromethyl-pyridine-2-carbonyl)-amin...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)C(F)(F)F)cc1C(O)=O Show InChI InChI=1S/C25H15F3N2O6/c26-25(27,28)15-7-8-29-20(11-15)22(31)30-19-9-13-3-1-2-4-14(13)10-21(19)36-16-5-6-17(23(32)33)18(12-16)24(34)35/h1-12H,(H,30,31)(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136430
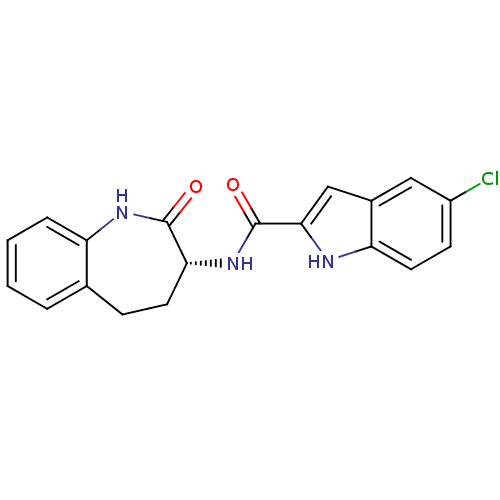
(5-Chloro-1H-indole-2-carboxylic acid ((R)-2-oxo-2,...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)N[C@@H]1CCc2ccccc2NC1=O Show InChI InChI=1S/C19H16ClN3O2/c20-13-6-8-15-12(9-13)10-17(21-15)19(25)23-16-7-5-11-3-1-2-4-14(11)22-18(16)24/h1-4,6,8-10,16,21H,5,7H2,(H,22,24)(H,23,25)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136450

(7-Chloro-1H-indole-2-carboxylic acid (1-methyl-2-o...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cccc(Cl)c2[nH]1 Show InChI InChI=1S/C19H16ClN3O2/c1-23-16-8-3-2-5-11(16)9-15(19(23)25)22-18(24)14-10-12-6-4-7-13(20)17(12)21-14/h2-8,10,15,21H,9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136414
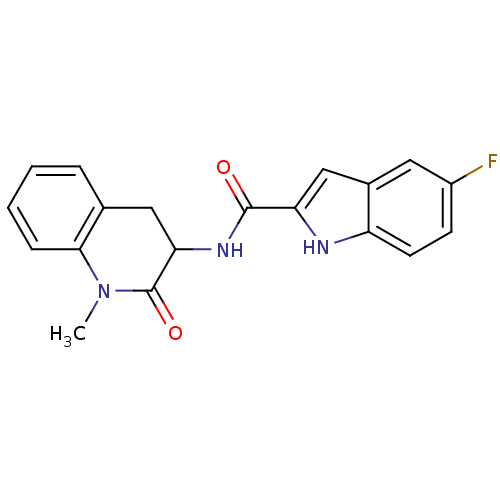
(5-Fluoro-1H-indole-2-carboxylic acid (1-methyl-2-o...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cc(F)ccc2[nH]1 Show InChI InChI=1S/C19H16FN3O2/c1-23-17-5-3-2-4-11(17)9-16(19(23)25)22-18(24)15-10-12-8-13(20)6-7-14(12)21-15/h2-8,10,16,21H,9H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
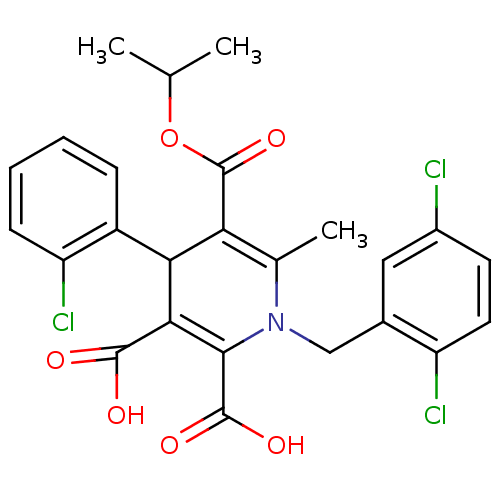
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133434
(4-(2-Chloro-phenyl)-1-(2,5-dichloro-benzyl)-6-meth...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2cc(Cl)ccc2Cl)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,23| Show InChI InChI=1S/C25H22Cl3NO6/c1-12(2)35-25(34)19-13(3)29(11-14-10-15(26)8-9-17(14)27)22(24(32)33)21(23(30)31)20(19)16-6-4-5-7-18(16)28/h4-10,12,20H,11H2,1-3H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136443
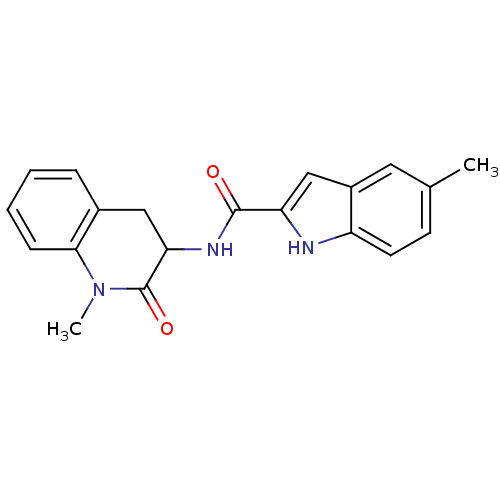
(5-Methyl-1H-indole-2-carboxylic acid (1-methyl-2-o...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cc(C)ccc2[nH]1 Show InChI InChI=1S/C20H19N3O2/c1-12-7-8-15-14(9-12)11-16(21-15)19(24)22-17-10-13-5-3-4-6-18(13)23(2)20(17)25/h3-9,11,17,21H,10H2,1-2H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136444

(5-Chloro-1H-indole-2-carboxylic acid (1-methyl-2-o...)Show SMILES CN1c2ccccc2CCC(NC(=O)c2cc3cc(Cl)ccc3[nH]2)C1=O Show InChI InChI=1S/C20H18ClN3O2/c1-24-18-5-3-2-4-12(18)6-8-16(20(24)26)23-19(25)17-11-13-10-14(21)7-9-15(13)22-17/h2-5,7,9-11,16,22H,6,8H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
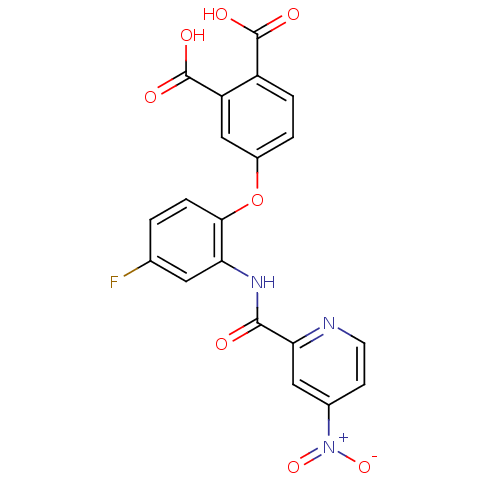
(Homo sapiens (Human)) | BDBM50135559
(4-{4-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2ccc(F)cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-10-1-4-17(32-12-2-3-13(19(26)27)14(9-12)20(28)29)15(7-10)23-18(25)16-8-11(24(30)31)5-6-22-16/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
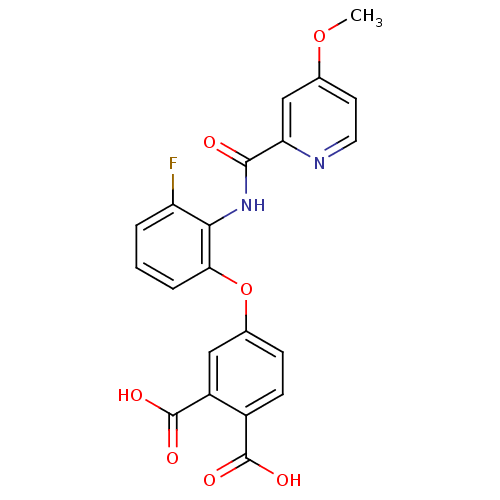
(Homo sapiens (Human)) | BDBM50135561
(4-{3-Fluoro-2-[(4-methoxy-pyridine-2-carbonyl)-ami...)Show SMILES COc1ccnc(c1)C(=O)Nc1c(F)cccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H15FN2O7/c1-30-11-7-8-23-16(10-11)19(25)24-18-15(22)3-2-4-17(18)31-12-5-6-13(20(26)27)14(9-12)21(28)29/h2-10H,1H3,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
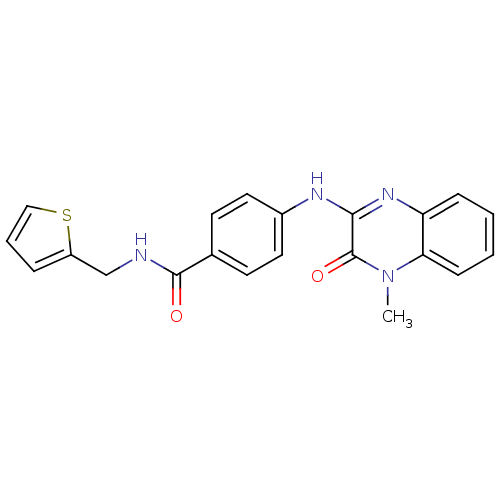
(Homo sapiens (Human)) | BDBM50172969
(4-(4-Methyl-3-oxo-3,4-dihydro-quinoxalin-2-ylamino...)Show SMILES Cn1c2ccccc2nc(Nc2ccc(cc2)C(=O)NCc2cccs2)c1=O Show InChI InChI=1S/C21H18N4O2S/c1-25-18-7-3-2-6-17(18)24-19(21(25)27)23-15-10-8-14(9-11-15)20(26)22-13-16-5-4-12-28-16/h2-12H,13H2,1H3,(H,22,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase of rabbit muscle |
Bioorg Med Chem Lett 15: 4790-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.021
BindingDB Entry DOI: 10.7270/Q2125TF8 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
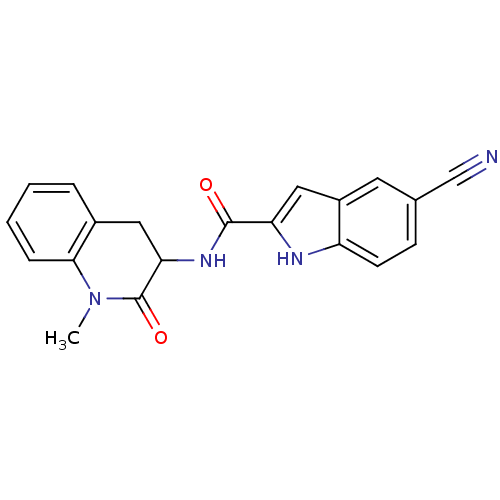
(Homo sapiens (Human)) | BDBM50136417
(5-Cyano-1H-indole-2-carboxylic acid (1-methyl-2-ox...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cc(ccc2[nH]1)C#N Show InChI InChI=1S/C20H16N4O2/c1-24-18-5-3-2-4-13(18)9-17(20(24)26)23-19(25)16-10-14-8-12(11-21)6-7-15(14)22-16/h2-8,10,17,22H,9H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50172978

(CHEMBL194177 | N-Isoxazol-3-yl-4-(4-methyl-3-oxo-3...)Show SMILES Cn1c2ccccc2nc(Nc2ccc(cc2)C(=O)Nc2ccon2)c1=O Show InChI InChI=1S/C19H15N5O3/c1-24-15-5-3-2-4-14(15)21-17(19(24)26)20-13-8-6-12(7-9-13)18(25)22-16-10-11-27-23-16/h2-11H,1H3,(H,20,21)(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase of rabbit muscle |
Bioorg Med Chem Lett 15: 4790-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.021
BindingDB Entry DOI: 10.7270/Q2125TF8 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50172972

(CHEMBL194807 | N-Furan-2-ylmethyl-4-(4-methyl-3-ox...)Show SMILES Cn1c2ccccc2nc(Nc2ccc(cc2)C(=O)NCc2ccco2)c1=O Show InChI InChI=1S/C21H18N4O3/c1-25-18-7-3-2-6-17(18)24-19(21(25)27)23-15-10-8-14(9-11-15)20(26)22-13-16-5-4-12-28-16/h2-12H,13H2,1H3,(H,22,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase of rabbit muscle |
Bioorg Med Chem Lett 15: 4790-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.07.021
BindingDB Entry DOI: 10.7270/Q2125TF8 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50136434
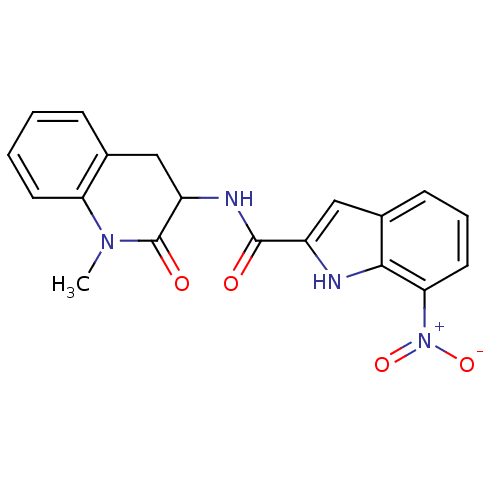
(7-Nitro-1H-indole-2-carboxylic acid (1-methyl-2-ox...)Show SMILES CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2cccc([N+]([O-])=O)c2[nH]1 Show InChI InChI=1S/C19H16N4O4/c1-22-15-7-3-2-5-11(15)9-14(19(22)25)21-18(24)13-10-12-6-4-8-16(23(26)27)17(12)20-13/h2-8,10,14,20H,9H2,1H3,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 4385-8 (2003)
BindingDB Entry DOI: 10.7270/Q2S75FQV |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133435

(4-(2-Chloro-phenyl)-1-ethyl-6-methyl-1,4-dihydro-p...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)OC(C)C |c:4,10| Show InChI InChI=1S/C20H22ClNO6/c1-5-22-11(4)14(20(27)28-10(2)3)15(12-8-6-7-9-13(12)21)16(18(23)24)17(22)19(25)26/h6-10,15H,5H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data